| page 1 | page 2 | page 3 | page 4 | page 5 |
Evan Lindquist, Artist-Printmaker |
Old Ink Appendix |
All information on this page was published by U.S. Department of Commerce, December 28, 1936, Circular of the National Bureau of Standards C413, "INKS", By C. E. Waters |
|
| Selected excerpts from Circular C413 are presented here. Major portions of the circular are not relevant to gallotannate inks, and I have not included them. Researchers who require authoritative information should consult the original publication. | |
Testing old inks |
|
THE TESTING OF INKS, pages 41-44, Circular C413 ... If the specification gives a formula for a standard ink, both the sample and standard are subjected to the same tests on the same sheet of paper. ... any dissimilarity between the standard ink and the sample becomes apparent at once. ... 1. IRON GALLOTANNATE INK ... The tests follow naturally from the definition of ink given by Schluttig and Neumann, which is quoted on an earlier page of this circular. The definition clearly tells what properties the ink should have, and tests are designed to find out whether the sample has these properties.... Because freshly made, well-settled ink should be clear, the first step in the examination of a sample is to allow the ink to remain undisturbed for 24 hours. If the sample is in several small bottles, which together contain the pint of ink called for in chapter five of Schluttig and Neimann, the contents of the bottles are poured into a single one of a suitable size.... [After the ink has settled for 24 hours, the bottle] is held up against the light and slowly tilted to see whether any sediment is at the bottom. There should be at most only traces of sediment. Very muddy ink can be reject without making any tests. ... The test for keeping-quality ... takes two weeks to complete. In two similar clear glass vessels ... [crystallizing dishes], place 25-ml portions of the sample and of the standard. The dishes, loosely covered, are kept ... in diffused daylight, but never in direct sunlight, for two weeks. At the end of this time, the sample should show no more surface skin than the standard, nor more deposit on the bottom and walls of the container. The iron content of the sample is determined in a 10-ml portion, by any suitable analytical procedure. The amount of iron in 100 ml of copying and record ink should not be less than 0.58 grams, nor more than 0.70 grams. For writing ink the limits are 0.29 and 0.35 grams. Streaks are made side by side on white bond paper with the sample and standard. The sheet of paper ... is pinned to a board or clamped to a pane of glass and held at an inclination of about 45 degrees. ... [The ink streaks must be made parallel to the "grain" of the paper.] If a piece of paper about an inch square is laid upon water,... it starts to curl up on opposite sides, thus making a shallow trough ... [which is parallel to the grain of the paper]. Measured portions, of about 0.6 ml each, of ink are allowed to flow down across the sheet of paper. The ink is measured in a pipette made of glass tubing of 3.5 mm (1/8 inch) bore, and approximately 250 mm (10 inches) long. A mark is etched or scratched approximately 60 mm (2 3/8 inches) from one end of the tube. These are only approximate measurements, because the exact volume of ink is of no great importance. The ends of the tube can be fire-polished, but they should not be constricted. Ink is drawn up to the mark, and kept from flowing out by pressing the tip of a finger against the upper end of the tube. While holding the tube vertically, its lower end is held against the upper edge of the inclined sheet of paper, the finger removed, and the ink is let flow out all at once and down across the paper. One or two more streaks are made with the sample ink and then, with another pipette, streaks of the standard, close beside those of the sample. The sheet of paper is left in position until streaks are dry, then put where it will be in diffused daylight, not in direct sunlight. It is not necessary, according to the specification, that the freshly made streaks of the sample and of the standard shall be exactly the same color, though they should be equally uniform in color. They should be of about the same shape and width, because these features indicate that the two inks are about equally fluid .... The streaks made with the sample should show no more evidence of striking through the paper than do those of the standard. After being kept in diffused daylight for one week, the streaks of the sample should be as intensely black (or blue-black) as those of the standard. The bottom half inch of the sheet is cut off and discarded. Then five strips, each about an inch wide, are cut from the lower end of the sheet. One strip is soaked in distilled water for 24 hours, the next strip is kept away from intense light and laboratory fumes. The third strip is soaked in a mixture of equal volumes of denatured alcohol and water for 24 hours. The fourth strip is also put away for later comparison, and the fifth is exposed at a distance of about 10 inches from a glass-enclosed carbon arc for 48 hours. When an arc lamp is not available, the test can be made by exposing the writing to direct sunlight, on the outside sill of a window facing south, but about double the number of hours will be required. In these three tests the sample should retain its color as well as does the standard. The comparison is made easier with the aid of the two strips, the second and fourth, that were set aside. Because of the temptation for the manufacturer to increase the amount of free mineral acid in his ink, so as to delay the deposition of sediment, a test of the corrosive action of the ink upon steel pens is made. This test is of no interest to the millions of users of fountain pens, because gold is not attacked by the acids that are in ink. The millions of users of steel pens must be looked out for. In the fiscal year beginning July 1, 1932, the Post Office Department alone asked for bids on 5,212,800 steel pens. The amount of metal dissolved from the pens is a rough measure of the acidity of the ink, and its determination may have some value in preventing the use of excessively acid ink for writing that must be kept for a long time. To make the corrosion test, take two new steel pens, for the same box, for each of the samples, and two for the standard ink. Rinse the pens with alcohol, then with ether, and dry them in an air-oven at 105 degrees Celsius (221 degrees Fahrenheit). Weigh each pair together to the nearest milligram. Because the preliminary washing is to remove the oily film from the metal, the pens should afterwards be handled with forceps. Immerse each pair of pens in 25 ml of ink, contained in a small beaker or flask, taking care not to have them "nested" together. After 48 hours, remove the pens, clean them with water and by rubbing to remove the tightly clinging deposit, rinse them with alcohol, and dry at 105 degrees Celsius. Again weigh, and if the loss in weight of the pair of pens in the sample is greater than the loss in the standard ink, the tests should be repeated with both inks and with new pens, to check the first results. Ink that contains oxalic acid usually forms on the pens a yellow crust of ferrous oxalate, which is not easy to remove by washing and scrubbing. To get rid of the crust, wash the pens with water and then, with forceps, hold them one at a time a few inches above a small flame, but do not heat them to redness. As soon as the crust blackens and ceases to smoke, drop the hot pens into water. It will then be comparatively easy to clean them in the regular way. The weight of metal dissolved depends to some degree on the surface area of the pens. This would not be true if corrosion stopped as soon as all the free hydrochloric or sulphuric acid was neutralized by dissolved iron. ... As a rule, all parts of the pens seem to be equally attacked by the ink, but now and then a strikingly different type of corrosion is met with. In this, the attack is chiefly at the edges, including those of the open slot and of the slits. Pens have been seen with their central slits opened to a width of nearly a millimeter. This effect, which seems not to have been mentioned in print elsewhere than in the immediate predecessor of this circular, is due to the ink, because both pens are always affected in the same way and to the same extent in a given ink. The effect has never been noticed with the standard ink. A possible reason is that the ink contains an acid that is barely able to dissolve iron, so its action is limited to the parts of the pens that have undergone the most severe mechanical treatment. This, of course, is where the metal has been cut. |
|
Dyes used in old ink |
|
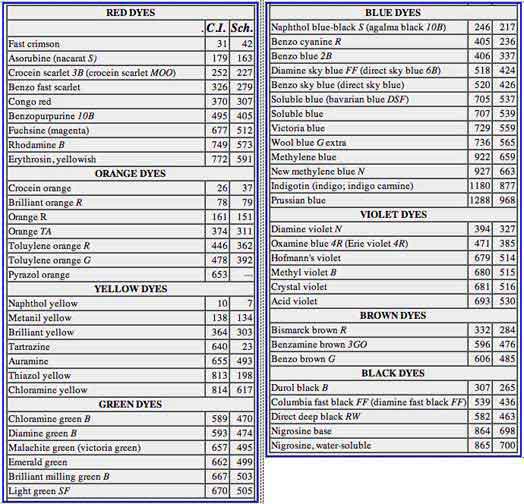
DYES FOR MAKING INK, pages 49-50, Circular C413 Not all dyes are equally suitable for making inks, and in the formulas in this circular, various dyes have been recommended. In each case the name of the dye is followed by certain letters and number in parenthesis, as "Soluble blue (C.I. 707; Sch. 539)." An explanation of these symbols is in order. There are many more dye names than there are different kinds of dyes because manufacturers like to use names of their own choice for their products. As a rule, the more widely a dye is used, the more apt it is to have a great many names. ... To do away with this sort of confusion, two important tabulations of dye names have been published. The first is Gustav Schultz's "Farbstofftabellen: (Dyestuff Tables), of which there have been several editions. The second is the "Colour Index" of the British Society of Dyers and Colourists. In each book the dyes are arranged in groups, according to the type to which they belong, and are further classified according to their chemical formulas in each group. ... Thus, 707 in the Colour Index means a particular dye. The same dye is no. 539 in Schultz's book. It will now be understood by the reader that such a symbol as (C.I. 707; Sch. 539) is the most certain way of telling the seller just what kind of dye is wanted. The Year Book of the American Association of Textile Chemists and Colorists gives the names by which the various types of dyes made in this country are known by the manufacturers. ... Many more dyes than those in the list [below] can be used for making inks. The list is given as an aid to readers who would otherwise have no idea what to ask for. [NOTE: this list was published in 1936 and may include names or numbers that are now obsolete.] The column heading "C.I." refers to the Colour Index of the British Society of Dyers and Colourists. The heading "Sch." refers to the classification by Gustav Schultz. |
|
|
|
In the two Federal specifications for iron gallotannate inks, the blue dye that must be used in the standard inks is the particular soluble blue designated as C.I. 707. According to the Colour Index, this dye is derived from a mixture of triphenylpararosaniline and diphenylrosaniline. These two dyes are insoluble in water, but dissolve readily when converted into the mixture of the trisulphonic acids, or of some of their salts. Because this conversion is never complete, the dye always contains some of the disulphonic, or even of the monosulphonic, acids. The acids are not used as such, but are converted into their sodium, ammonium, or calcium salts. The calcium salts of the mono- and di- acids are nearly insoluble in water, and for that reason some manufacturers make the calcium salts when the dye is for use in ink. For two reasons it is less profitable to sell the calcium instead of the sodium salts. First, the mono- and disulphonic acids cannot be sold mixed with the trisulphonated product. In the second place, considering the atomic weights and valences, a given weight of the acid will yield a somewhat greater weight of the sodium than of the calcium salt. Schluttig and Neumann used bavarian blue DSF, which is the sodium salt of the disulphonic acid of triphenylpararosaniline, mixed with more or less of the monosulphonate. Both tannin and iron salts are used as mordants in dyeing. That is, they form insoluble compounds with some kinds of dyes, and thus serve to fix them upon the fabric. Because of the composition of iron gallotannate inks, by no means all classes of dyes can be used in them. The dye must be of a type that does form an insoluble compound with anything else in the ink. Also, because sometimes a factory batch of soluble blue is not sulphonated as it should be, it is advisable when buying to specify that the dye must be "for ink". A reliable dealer will then not supply soluble blue that is satisfactory for ordinary uses, but not for making ink. The new formulas in section II, 5 (e) require exceptionally good dye, and there may be some difficulty in getting it. Unfortunately there are no laboratory tests, aside from making ink and testing it, by which it can be determined whether the dye is of the desired quality. With suitable dye, the new inks should show greater stability in the sediment test than the standard blue-black writing ink. A few dyes that are more or less satisfactory substitutes for soluble blue are naphthol blue-black S (C.I. 246; Sch. 217), benzo blue 2B (C.I. 406; Sch. 337), diamine sky blue FF (c.I. 518; Sch. 424), and benzo sky blue (C.I. 520; Sch. 426). Acid black N (C.I. 294; Sch. 261), when used at the rate of 3.5 g in a liter, has nearly the same shade of color as soluble blue. Two dyes that caused rapid deposition of sediment were durol black B (C.I. 307; Sch. 265) and direct deep black RW (C.I. 582; Sch. 463). These remarks apply only to the use of the dyes in iron gallotannate ink. The dyes have not tested in iron gallate ink. In addition to bavarian blue, Schluttig and Neumann named three other dyes which they used for matching the exact shades of inks submitted to them for test. Apparently these inks had a wide range of colors, because they used red, brown, and green dyes. Their red dye was azorubine, which they called nacarat S (C.I. 179; Sch. 163). The green dye was guinea green B (C.I. 666; Sch. 502), which they knew as acid green VBSPo. Their "Kastanienbraun" (chestnut brown) cannot be found in the Colour Index nor in Schultz's Farbstofftabellen. The chestnut brown of the Colour Index is umber, not a dye at all, but an insoluble earth used as a pigment. In making dyes, it is often necessary to salt them out of solution; that is, to precipitate them by dissolving common salt in a concentrated solution of the dye. When this must be done, the dye unavoidably contains more or less salt. Many dyes also are intentionally mixed with salt or some other uncolored substance to dilute them to the strength with which dyers have long been familiar, because the formulas furnished by manufacturers are based upon these diluted dyes. This is a recognized trade practice that is not to be regarded as adulteration. For making inks it is preferable to have the concentrated forms of the dyes, and these should be ordered from the manufacturer, even though they cost more. |
|
Literature on inks |
|
| This circular gives only a few of the very numerous published formulas for inks. Every public library has books in which other formulas can be found.... [NOTE: All references cited here are from the circular, published in 1936]. | |
| 1. A.H. Allen, Commercial Organic Analysis, 5th ed., 5, 205-267 (P. Blakiston's Son & Co., Philadelphia, 1927). 2. H.Bennett, Practical Everyday Chemistry (The Chemical Publishing Co., New York, NY, 1934). 3. H.Bennett, The Chemical Formulary, 3 vol. (D. Van Nostrand Co., New York, 1933-1936). 4. W.T. Brannt and w.H. Wahl, Techno-Chemical Receipt Book (H.C. Baird & Co., Philadelphia, 1905). 5. D. Carvalho, Forty Centuries of Ink (Banks Law Publishing Co., New York 1904). 6. Henley's Twentieth Century Book of Recipes, Formulas, and Processes (Norman W. Henley publishing Co., New York, 1928). 7. J.B. Lavay, Disputed Handwriting (Harvard Book Co., Cambridge, Mass., 1909). 8. S. Lehner, Manufacture of Inks (Translated, with additions, by W.T. Brannt, H.C. Baird & Co., Philadelphia, 1892). 9. C.A. Mitchell and T.C. Hepworth, Inks, Their Composition and Manufacture, 3d ed. (Chas. Griffin & Co., (Ltd.), London, 1924). 10. A.S. Osborn, Questioned Documents (Lawyers' Cooperative Publishing Co., Rochester, NY, 1910). 11. J.H. Oyster, Spatula Ink Formulary (Spatula Publishing Co., Boston, 1912). 12. O. Schluttig and G.S. Neumann, Die Eisengallustinten (The Iron-Gall Inks) v. Zahn & Jaensch, Dresden, 1890). 13. Scientific American Cyclopedia of Formulas. Edited by A.A. Hopkins (Munn & Co., New York, 1921). 14. E. Spon, Workshop Receipts (E. & F.N. Spon, London and New York, 1917). 15. N. Underwood and T.V. Sullivan, Chemistry and Technology of Printing Inks, (D. Van nostrand Co., New York, 1915). 16. F.B. Wiborg, Printing Ink (Harper & Bros., New York, 1925). |
|
| Articles in Chemical Journals are: | |
| • L.S. Munson, The Testing of writing inks, J. Am. Chem. Soc. 28, 512-516 (1906). • F.F. Rupert, Examination of writing inks, Ind. Eng. Chem. 15, 489-493 (1923). • E.W. Zimmerman, Colored waterproof drawing inks. Ind. Eng. Chem. 25, 1033 (1933). • C.E. Waters, Blue dye as evidence of the age of writing, Ind. Eng. Chem. 25, 1034 (1933). |
|
| A few publications of the National Bureau of Standards relate to inks. The Federal specifications, formerly issued as circulars of the Bureau, are now part of the Federal Standard Stock Catalog, and are no longer distributed by the Bureau. Many public, college, and university libraries throughout the country have the publications of the Bureau.... The letter circulars mentioned below are mimeographed, and are not handled by the Superintendent of Documents ... [NOTE: Some of the circulars listed below may have been in the nature of ephemera and may be very difficult to locate. The "Out of Print" notation indicates that the circular was no longer available when this list was published in 1936.] |
|
| • J.B Tuttle and W.H. Smith, Analysis of Printing Inks, BS Tech. Pap. 39 (1915). (Out of Print.) • Composition, Properties, and Testing of Printing Inks, BS Cir. 53, 1915. (Out of Print.) • Inks--Their Composition, Manufacture and methods of Testing. Cir. BS C95, 1st ed. (1920). (Out of Print.) • Inks, Typewriter Ribbons and Carbon Paper. Cir. BS C95, 2d ed. (1925). (Out of Print.) • Inks, Cir. BS C400 (1933). (Superseded by C413.) (NOTE: The predecessor to this circular.) • P.H. Walker, Some Technical methods of Testing Miscellaneous Supplies. Misc. Pub. BS M15 (1916). A reprint, with notes and corrections, of Bur. Chemistry, Dept. Agr. Bul. 109, (1912). The methods differ considerably from those of the federal specifications. (Out of Print.) • A.E. Kimberly and B.W. Scribner, Summary Report of Bureau of Standards Research on Preservation of Records. Misc. Pub. BS M144 (1934). (Out of Print.) • E.W. Zimmerman, C.G. Weber, and A.E. Kimberly, Relation of ink to the preservation of written records. J. Research NBS 14, 463-468 (1935), RP779. 5c. • E.W. Zimmerman, Iron gallate inks -- liquid and powder. J. Research NBS 15, 35-40 (1935) RP807. 5c. • C.E. Waters, Inks for recording instruments. J. Research NBS 17, 651 (1936) RP935. 5c. • Dry Etching of Glass. BS Letter Circular LC150. Free. • Carbon Paper and Typewriter Ribons. BS Letter Circular LC424. Free. • Stain Removal from Fabrics: Home Methods. U.S. Dept. Agr. Farmers' Bul. 1474 (1930). 5c. • B.L. Wehmhoff, and D.P. Clark, Standard mimeograph Ink and Paper. U.S. Govt. Printing Office Tech. Bul. 15 (1932). Free. • B.L. Wehmhoff, and D.P. Clark, and D.H. Boyce, Newsprint and news Ink, U.S. Govt. Printing Office Tech. Bul. 18 (1933). Free. |
|
| The following Federal specifications are sold separately by the Superintendent of Documents for 5c apiece (in 1936): | |
| • TT-I-521, Ink; copying and record. • TT-I-528, Ink; drawing, waterproof, black. • TT-I-531, Ink; drawing, waterproof, colored. • TT-I-542, Ink; marking, indelible (for) fabrics. • TT-I-549, Ink; red. • TT-I-556, Ink; stamp-pad • TT-I-563, Ink; writing. |
|
WASHINGTON, October 2, 1936 |
|
ACKNOWLEDGEMENTS Thanks to the following friends: To Art Maier, Fulton, Missouri, for providing his copy of Circular C413 from which this information is taken. To Alex Carter, Epping New South Wales, for submitting additional information and for his extraordinary proofreading skills. |
|
--Evan Lindquist |
|
This is the last of five pages about gallotannate inks. |
|
| Click signature at bottom of any page to return to Top |